Introduction To Module 2.5 Clinical Overview
Let the Snow Regulatory Affairs Consulting team assist your team in Module 2.5 submission preparation
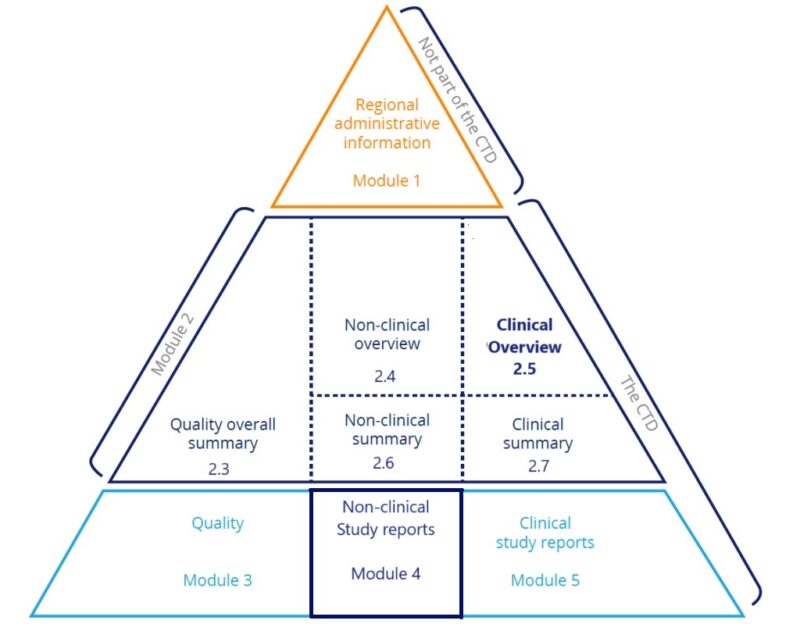
The introduction to Module 2.5 typically encompasses the following sections:
- Purpose and Scope: This section outlines the purpose of the clinical overview and the scope of the included clinical data, defining the objectives of the clinical trials and the target population for the product.
- Regulatory Context: An overview of the regulatory requirements for clinical data submission is provided, including relevant guidelines and regulations governing the conduct and reporting of clinical trials.
- Product Description: A brief description of the drug or medical product is given, detailing its indication, formulation, dosage form, and proposed clinical use.
- Summary of Clinical Development Program: This section summarizes the entire clinical development program, detailing the phases of clinical trials conducted, study designs, patient populations studied, and primary endpoints evaluated.
- Key Efficacy and Safety Results: An overview of the key efficacy and safety results from clinical trials is presented, highlighting significant findings, trends, and outcomes that support the product’s efficacy and safety profile.
- Summary of Benefit-Risk Assessment: A concise assessment of the benefit-risk profile of the product is provided based on the available clinical data. This section may discuss the clinical significance of the observed efficacy and safety outcomes, as well as any potential risks associated with the product’s use.
Connect with Snow Regulatory Affairs Consulting
 In summary, partnering with Snow Regulatory Affairs Consulting to navigate the FDA PSP/IPSP process can greatly enhance your drug development strategy. Our specialized knowledge, strategic planning, and ability to streamline interactions with the FDA will not only expedite the process but also increase the chances of a successful outcome, ultimately bringing your product to market faster and more efficiently. So give us a call today at (714) 553-3736 or visit us online at regulatoryaffairsconsultants.com. For further information or to discuss how I can assist with your specific needs, please feel free to contact me. Together, we can navigate the regulatory landscape and bring innovative treatments to pediatric patients in need.
In summary, partnering with Snow Regulatory Affairs Consulting to navigate the FDA PSP/IPSP process can greatly enhance your drug development strategy. Our specialized knowledge, strategic planning, and ability to streamline interactions with the FDA will not only expedite the process but also increase the chances of a successful outcome, ultimately bringing your product to market faster and more efficiently. So give us a call today at (714) 553-3736 or visit us online at regulatoryaffairsconsultants.com. For further information or to discuss how I can assist with your specific needs, please feel free to contact me. Together, we can navigate the regulatory landscape and bring innovative treatments to pediatric patients in need.
Click here to engage with our regulatory experts and explore with us questions you may have.
It All Starts With a consultation!
We look forward to discussing your needs with you!
For any kind of Regulatory Affairs inquiries, Please call
tim@gotresults.net
