Investigational New Drug Applications and Submissions
Snow Consulting will make sure your IND application meets FDA’s stringent IND filing requirements and necessary components.
What is an IND?
An IND, Investigation New Drug Application is a request for authorization submitted by you, the Sponsor to the FDA in order that the Sponsor can conduct clinical trials for unapproved drugs. The purpose of an IND application is to allow Sponsors to begin conducting clinical trials on humans, and approval to ship the drugs across state lines in order to conduct these trials.
Types of INDs
The 2 Categories of INDs are:
Commercial IND: Commercial INDs are used when the Sponsor intends to bring the drug to the open market. This means commercial INDs can apply to drugs created by non-profit groups who intend to eventually put the drug on the open market. The application process and timeline for a Commercial IND is much longer and more complex than for a Research IND.
Research IND: Research INDs are used when the intention is to prove efficacy for a new indication of an already approved drug. Research IND applications are submitted by physicians, and with a process that is less complex than Commercial INDs. For example, Research INDs typically involve fewer investigators and are often done at a single testing site.
How to Submit an IND – Streamlining Your IND Application Process with Expert Guidance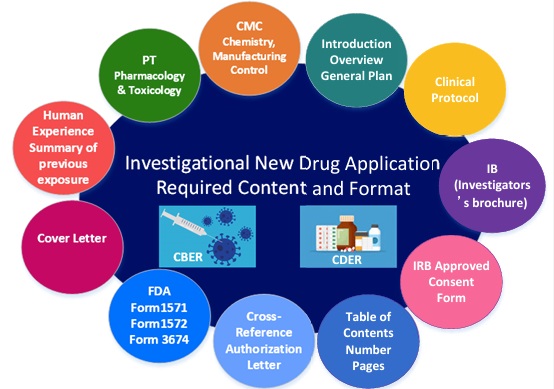
When seeking FDA authorization to administer an investigational drug or biological product to humans, your IND (Investigational New Drug) application is crucial. Approval of this application is mandatory before transporting your product across state lines to clinical sites. The intricacies involved in submitting an IND can be daunting and challenging to navigate.
For instance, Commercial IND applications must be filed via the FDA’s Electronic Submissions Gateway, while Research IND applications can be submitted in paper form to the appropriate address. This highlights just one of the many complexities in the IND application and submission process.
Given these challenges, partnering with an experienced FDA consultant is invaluable. At Snow Consulting, we are committed to ensuring that your IND application aligns with the FDA’s rigorous filing requirements and includes all necessary components. Our expertise will help you stay prepared and on course throughout the submission process.
IND Filing Requirements
Each IND application should include the following:
- Form FDA 1571 (IND application cover letter)
- Form FDA 1572 (Investigator’s statement)
- Form FDA 3674 (certification requirement & mandatory registration and reporting of results for applicable clinical trials through ClinicalTrials.gov.)
- Table of contents
- Introductory statement
- General investigational plan
- Investigator’s brochure
- Protocol(s): study protocol(s), investigator data, facilities data, Institutional Review Board (IRB), Chemistry, manufacturing, and control (CMC) data, including environmental assessment or claim for the exclusion (assuming the draft of CMC information exists)
- Preclinical: pharmacology and toxicology data
- Clinical: previous human experience
- Additional information to support the IND filing
Together with you, we will meticulously and carefully review the IND application. Our team will provide you with the final copy that is FDA ready for your Regops to submit. The IND application will go into effect 30 days after the FDA receives the application unless it was subject to a clinical hold from the FDA. Sometimes, the agency approves it earlier and notifies the Sponsor that clinical trials can begin.
 So let Snow Consulting “guide you” or help with your IND application or the part that you need, give us a call, 714-553-3736.
So let Snow Consulting “guide you” or help with your IND application or the part that you need, give us a call, 714-553-3736.
It All Starts With a consultation!
We look forward to discussing your needs with you!
For any kind of Regulatory Affairs inquiries, Please call
tim@gotresults.net
